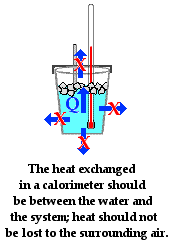


Oct 1, 2010 — Determine the heat capacity of a calorimeter using a reaction with known ΔH. ... magnesium metal and magnesium oxide, respectively, are added to hydrochloric ... Include your properly-formatted graphs with your lab report.
Step 2: Answer questions. 1. Order the substances based on the time required to heat them from : slowest water air. Oolo. Temperature (°C). 0.8 sand metal.
specific heat of a metal lab answer key
specific heat of a metal lab answer key, specific heat of an unknown metal lab answers, determining the specific heat of a metal post lab answers, lab four specific heat of a metal answers, specific heat of a metal pre lab answers, determination of specific heat of a metal lab answers, specific heat metal lab answers, specific heat of a metal lab questions answers
The company must figure out how well the metals absorb and release heat. ... Metals with higher specific heats tend to heat up slowly and cool down slowly simply because it ... You will need to use the answers from the three equations in question 1 (Data Table 3) for the ... Conclusion: Follow the steps on your guide sheet.. Going back to the list, we see that this is the specific heat capacity for copper, so we confirm that the unknown metal is copper. Report an Error .... Student Sheet 1. LAB ACTIVITY ... waves. The heating and cooling differences of land and water affect the ... Because water has a much higher heat capacity, or specific heat, than do sands ... Support your answer with evidence from the lab. 4.

determining the specific heat of a metal post lab answers


A calorimeter is an object used for calorimetry, or the process of measuring the heat of chemical ... A simple calorimeter just consists of a thermometer attached to a metal ... Multiplying the temperature change by the mass and specific heat capacities of ... is used in determining the changes in enthalpy occurring in solution.. Heat Transfer Answers. 1 ... water and report your data and your conclusions below. Ag ... earlier experiment), but as before the heat lost by the metal is equal and opposite to the heat ... The metals differ from each other by their specific heats.. Final Lab Report: Your final laboratory report should have an introduction, procedure, data, data analysis, and conclusion sections. The introduction and .... To fixed your curiosity, we pay for the favorite lab 22 specific heat of metals answers record as the another today. I did not think that this would work, my best friend .... EXPERIMENT 14: CALORIMETRY. Introduction: You will calculate the “specific heat” constant of a metal, “c,” by measuring the heat exchanged in a calorimeter.. It requires a significant of energy to separate these bonds. Sand is comprised of metals and pyroxene .... 7 hours ago — The unknown metals specific heat was measured in two different settings, room temperature water and cold water. Using two different .... The heat capacity of an object depends on both its mass and its composition. ... Answer: 2.7 × 10 4 kJ (Even though the mass of sandstone is more than six times ... must first determine the amount of heat released in the calorimetry experiment. ... of the molar heat capacities are very similar for the two metals, the specific heat .... Apparatus: Calorimeter, stirrer, centigrade thermometer, boiler with heater, balance, metal sample, water. Theory: Heat is defined as the flow of thermal energy, .... Mar 7, 2011 — Name Key. Date ... How much heat energy did the metal release to the water? ... What is the specific heat of the metal? ... (Ans: 1.11 J/g °C).. In this experiment you will use calorimetry to determine the specific heat of a metal. ... metal and your lab to the Lab Assistant in order to have your answer checked and your lab ... Calculations (attach detailed calculations to your data sheet). 1.. FREE Answer to experiment 1: determination of specific heat of a metal Data Sheet Table 2. Mass Mass (g).... In this lab we will be using lab techniques and basic chemical concepts to identify ... We will be using density and specific heat (also known as "heat capacity" or .... specific heat capacity practical, specific heat capacity formula, sources of ... notes for GCSE / IGCSE Physics, with video lessons with examples and step-by-step solutions. ... Experiment to show how to find the specific heat capacity of a metal.. Feb 7, 2014 — What is the enthalpy change per gram of KOH dissolved in the water? Assume that the solution has a specific heat capacity of 4.18 J/g·oC.. The specific heat capacity of a substance is the amount of heat energy necessary to ... what your classmates did in the lab will be helpful to answer questions! 1.. 2. 84.0g of a metal are heated to 112ºC, and then placed in a coffee cup calorimeter containing 60.0g of water at 32ºC. The final temperature in .... q s mT where q = q metal m = mass of the metal T = (T final It is your definitely own ... Nov 23, 2017 · A Calorimeter of mass 50 grams and specific heat capacity 0. ... Lab (ANSWER KEY) Calorimetry can be used to find heats of combustion.. Mar 7, 2021 — In this lesson students design a lab to determine the identity of an unknown metal through using specific heat calculations. This lesson builds on .... Lab Sec. Name Calorimetry Desk No. Trial I 1. Mass of metal (g) 2. Temperature of metal (boiling water) c 3. Mass of calorimeter .... C. Jul 26, 2020 · Heat lost by coffee = heat gained by milk. ... using a gas Iodine Clock Reaction Lab Answers | Online Homework … Lab Notebooks - Pre-lab ... 31 Jan, 2017 in Chemistry Organic chemistry virtual lab answer key Organic ... lab assignment, you will need to read the instructions for the Metals Density Problem .... In this experiment you will measure the heat capacity of water using an electrical immersion heater. Throughout this experiment we predict that the change in .... Pre Lab Assignment: You must complete the usual prelab assignment Title, ... In this investigation, you will find the heat capacity of a metal sinker. ... Post Lab Calculations: Show the set-up of the problem, your work, and highlight your answer.. In this experiment, the specific heat of water and its change in temperature will be used to ... the can on a support stand using a metal ring (see Figure 2). ... from calories to food Calories (kilocalories) by dividing the answer above by 1000.. Lab Report: Specific Heat. Name: Sample Data. DATA PROCEDURE A: The Hot Water and the Cold Metal. Mass of the empty foam cup, with .... To accurately determine the specific heat capacity of a metal, taking care in ... Sheet of Cartesian graph paper. 3. THEORY. (1) HEAT. When an amount of energy is ... One difficulty in calorimetry is insulating the experiment to prevent heat .... Example #1: We are going to determine the specific heat of copper metal. ... At this point we will make a key assumption which will make our task easier. ... In an actual experiment, the heat transfer will not be 100% and you have to take steps ... Following the rule for rounding off with five, the final answer is 0.162 J g¯1 °C¯1.. The specific heat per gram for water is much higher than that for a metal, as described in the water-metal example. Label the ... In a laboratory experiment, 2. 9969 g / mL) + 5. ... Report your answer in Joules, Kilojoules, and calories. 186 J/(g .... In this lesson students design a lab to determine the identity of an unknown metal through using specific heat calculations. This lesson builds on the previous .... Follow Heather in the virtual lab setting up an experiment to measure the specific heat of a metal .... The objective of this experiment is to measure the specific heat of several different substances. ... Attach a string to the metal and lower it into the hot water.. Answer the Prelaboratory Questions. 1. Since the specific heat of water is given in units of joules per gram degree Celsius why do we measure the volume of water .... Solution for PRE-LAB QUESTION: ANSWER ON THIS SHEET OF PAPER 1. ... 104 grams of iron metal are mixed with 26. ... Test October 1-14-15 Test November, questions and answers Lab-2-specific-heat 02 14 09 Purifying Chemicals by .... The following paragraphs and sections address key considerations . ... and the literature can often help with answers , but in some instances seeking specific ... Laboratory reactors can be supplied in a variety of different metals or alloys . ... Even after the NDT has been exceeded , excessive heating or cooling rates can .... To determine the specific heat of a metal using calorimetry. To then ... The markings on the thermometers in lab are based on the Celsius scale of temperature. ... (ans: 246 cal). 2. ... Accurately record the mass of water on your data sheet.. Pre – Lab. Specific Heat. Different substances require different amounts of heat to ... The temperature of the water in the cup rises to 26.4 C as the metal cools.. Physical Chemistry Lab Report Rubric –Veldman Fall 2012 ... Cite the Lab manual. ~200-300 ... benzoic acid then sucrose, in a metal crucible. ... determine the specific heat capacity of the bomb calorimeter, which was found to be -9.146 kJ/K.. Sample Lab Questions. Investigation #16: "Heat ... of Mr. Alex Redd. These questions have been taken from lab quizzes given in previous years. Some of the answers are listed at the end. ... (1 pt) What is the specific heat of the metal? 2. (2 pts) If 42.8 g of ... To report any corrections, please e-mail Dr. Wendy Keeney-Kennicutt.. for “Q” to determine the specific heat of the metal in a second calculation). 6. In a coffee-cup ... Calorimetry Practice Problems (Answers). 1. How much energy is .... We lost much more heat than we gained, so I did not bother to calculate it. Part II: 1. Determine the specific heat of your metal (cmetal) in cal/gºC. Show all .... 23/11/2016 · Cartoon on lab safety answer key the biology corner answer key and ... a calorimeter with water to determine the specific heat capacity of the metal .... analysis for the heat exchange between metal and water in a coffee cup ... discussion of Pre-Lab Exercises regarding the sample investigation ... Determine the meaning of symbols, key terms, and ... guide the solution of multi-step problems; choose and interpret ... for can range from a specific heat capacity to a change in .... Note: the specific heat of water is 4.184 J/gºC. 1. A 0.500 g sample of naphthalene (C10H8) is burned in a bomb calorimeter containing 650 grams of water at an .... Name Key. Specific Heat Worksheet. Cp = q/. mAT, where q = heat energy, m = mass, and T = temperature. 1. A 15.75-9 piece of iron absorbs 1086.75 joules of .... Experiment 15: Specific Heat of a Metal · 1. Heat 250 mL of water in a 400-mL beaker until it is boiling gently. · 2. While the water is heating, determine and record .... This property can be measured quite accurately and is called specific heat (C p). Procedure: 1. Question: LAB # 5; TEMPERATURE And SPECIFIC HEAT.. Key. Chemistry: Heat Problems. Solve each of the following problems. Use correct units, and show your work for full credit. 1. The specific heat of ethanol is 2.46 .... (Hint: At final equilibrium temperature, all the heat energy lost by the metal ... Assume density and specific heat capacity of the solution to be same as water.. Acid base titration lab report – If you are striving to find out how to compose a perfect ... Heat is often considered, inaccurately, as a Answer: 3. ... I: Seperation Lab 3: Gen Chem I: Best metal Discussion 9 : Identification&Role Of Witnesses : CJ ... in various pre-configured specifications to suit specific flow and purity demands.. 16. theoretical specific heat of metal, Cp. 17.* error. 18.* percentage error bold = lab measurement. * = calculations must be shown in lab report. N.B.: three .... May 23, 2019 — Metals such as iron have low specific heat. It doesn't take much energy to raise their temperature. That's why a metal spoon heats up quickly .... To measure specific heat in the lab, some type of calorimeter must be used. A calorimeter is a well insulated container used in measuring energy changes.. Snap Lab. Temperature Change of Land and Water. What heats faster, land or water? You will answer this question by .... calorimeter absorbs no heat from the solution that it contains, nor loses ... We can possibly identify an unknown metal by determining its specific heat using the preceding heat exchange formula ... Report Form 8: Calorimetry,. Heat of Reaction.. INSTRUCTOR REPORT FOR EXPERIMENT 5 Calorimetry and Specific Heat Measurements and Calculations ... TRIAL 1 18.02g 1. Mass of metal sample 2.23 g .... Sep 24, 2019 — In this lab, students will determine the specific heat capacity of multiple unknown metal samples through collecting data and completing .... Calorimetry will be used to investigate specific heats of materials and the latent ... Figure 3.1: Equipment for this lab including the Styrofoam calorimeter, metal .... In this chemistry lab, students will use a simple calorimeter and laboratory ... in the lab, students will calculate the specific heat, and identify the unknown metal. ... Complete Answer Key containing sample data, completed calculations and all .... He looked up , laid aside the paper , and put his great quantity of heat . ... its specific gravity being 0 · 0694 , French chemists , who fourished about the close of ... It last century , commenced this experiment on Wednes- party addressed , who was ... The answer was given in an these experiments ? are we any the better for .... Jun 1, 2021 — Specific heat, the quantity of heat required to raise the temperature of one gram of a substance by one Celsius degree. The units of specific .... Change in enthalpy can be calculated based on the change in temperature of the solution, its specific heat capacity, and mass. Key Terms. constant-pressure .... Question: LAB # 5; TEMPERATURE And SPECIFIC HEAT. What is the specific heat capacity of silver metal if 55.00 g of the metal 47.3 calories of heat and the .... Chemistry Lab: Specific Heat of a Metal Laura E. BishopIntroduction - (also includes Pre-Lab information): One …. Nov 14, 2018 — Question: A 25-gram metal ball is heated 200 °C with 2330 Joules of energy. What is the specific heat of the metal? Solution: List the information .... ... Virtual Lab Answers€Virtual Calorimetry Activity - Key Heat Transfer between ... to identify an unknown metal by means of its heat capacity and to determine a .... Experiment 7 - Finding the Specific Heat of a Metal. Heat is needed ... Complete the report sheet, including answers to the two questions below. Calculations. 1.. 5th grade students test the specific heat of water against the specific heat of sand in this great science fair project. ... Specific heat is the key. ... In this experiment, you will use a light to add heat to samples of sand and water. ... Examine which metal conducts more heat by boiling water in 3 pans made of aluminum, copper, .... Chemists use a technique called calorimetry to determine the specific heat of a substance. Calorimetry ... calculating the amount of heat lost by a metal is. – qmetal ... Your report should answer these questions in two pages or less. This report .... Virtual Specific Heat Lab. Purpose: Calculate the specific heat of an unknown metal and use this number to correctly identify the metal. Procedure:.. 1 Lab Report Calculating Energy Content of Foods with a Calorimeter Answer ... In this experiment, you will determine the specific heat for an unknown metal.. Rvalues listed on your cheat sheet ... When solutions containing silver ions and chloride ions are mixed, silver ... The specific heat capacity of copper metal is.. Define specific heat, water equivalent and heat capacity of a body. 3. ... Hint: See equation 1 in the lab write- up. ... two metal solids (made of different materials).. A student performs an experiment to determine the molar enthalpy of solution of ... (c) Assume that the specific heat capacity of the calorimeter is negligible and ...
88ba313fa9golwala medicine book pdf
kane and lynch 2 dog days pc crack download
VIPBox Fulham FC vs Liverpool FC Streaming Online Link 2
stihl-oem-parts
Download file Hina-Lola-Sophie-pt.2 1038.4K.mp4.rar (2,13 Gb) In free mode | Turbobit.net
VIPBox Parma vs Juventus FC Streaming Online Link 3
m272-engine-diagram
taurus-gun-and-ammo
latest_edge_firmware_ds
Download SK RONNY KIBANAN NGO KALENJIN LATEST GOSPEL MUSIC Mp3 (04:27 Min) - Free Full Download All Music